
OVERVIEW
We focus on understanding the molecular aspects of chromatin regulation and have a long-standing interest in the study of histone variants. We want to find ways to translate knowledge about chromatin regulation into therapeutic tools for the management of diseases such as blood cancers.
OUR RESEARCH
We seek to bridge the gap between basic molecular research and translational research by exploring chromatin regulation, in particular the molecular biology of histone variants. We aim to exploit this knowledge for the identification of novel intervention strategies for the treatment of blood cancers. We focus on the continuum of myeloid diseases, ranging from the premalignant expansion of altered clones to chronic myelodysplastic syndromes and acute myeloid leukaemia.
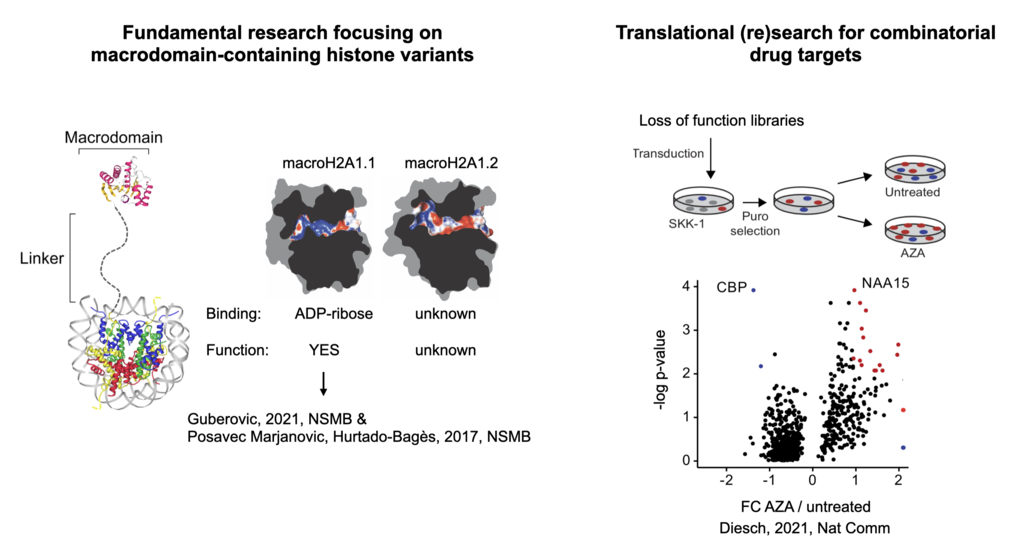

OUR GOALS
Through our research, we aim to gain a better understanding of the epigenetic mechanisms that contribute to the development of blood cancers. By functionally mining the chromatin regulatory space, we further aim to provide new starting points by identifying novel drug targets. In this regard, our research focuses on two main lines:
1. To mine the chromatin regulatory space to identify novel drug targets that can either help improve current treatments or intercept disease at an early asymptomatic stage.
2. We study histones from the protein core of the nucleosome, particularly the variant macroH2A that led to two major discoveries: its major role in nuclear organization and its ability to bind metabolites through its mostly understood macrodomain, establishing a direct link between chromatin and metabolism.
OUR CHALLENGES
Through our research, we hope to answer the following questions:
1. How do epigenetic mechanisms operate on the molecular level?
2. How do chromatin and, in particular, histone variants contribute to cell fate transitions?
3. How can we exploit this knowledge for the development of novel therapeutic strategies?
Team

Marcus Buschbeck
Senior group leader
Senior group leader

David Corujo
Postdoctoral Investigator
Postdoctoral Investigator

Florencia Lucia Herbstein
Postdoctoral Investigator
Postdoctoral Investigator

Jeannine Diesch
Postdoctoral Investigator
Postdoctoral Investigator

Marina Farkas
Postdoctoral Investigator
Postdoctoral Investigator

René Winkler
Postdoctoral Investigator
Postdoctoral Investigator

Bedri Batuhan Yaman
PhD Student
PhD Student

Jonathan Blickenberger
PhD Student
PhD Student

Mehwish Rehman
PhD Student
PhD Student
Nadya Christina
PhD Student
PhD Student

Paula Roquero
PhD Student
PhD Student

Shubhra Ashish Bhattacharya
PhD Student
PhD Student

Valentina Valka
PhD Student
PhD Student

Vanesa Valero
Medium Lab Technician
Medium Lab Technician

Sonia Nuñez
Project Manager
Project Manager
Selected Publications
Current Grants
CI24-10180
Fundació "la caixa"
DegradAML Molecular glue degraders for a novel drug target in acute myeloid leukemia
Centre national de la recherche scientifique
EpiGene3Sys Moving Epigenetics towards Systems Biology – EpiGene3Sys.

PRYGN222668BUSC
Fundación científica de la asociación española contra el cáncer
EPISTROMA Re-educación epigenética del estroma en el microambiente de la médula ósea como enfoque terapéutico en la prevención de cáncer de sangre

PID2024-161430NB-I00
Ministerio de ciencia, innovación y universidades
MacroGenomics Gene regulatory functions of macrodomain-containing histone variants and their impact on cellular and animal physiology
101166838
European commission
NUCLEAR METABOLIC REGULATION OF GENOME FUNCTION AND CELL IDENTITY
101168783
European commission
HubMOL Hub Molecules of Metabolism and Signalling – Key regulators of Life
101126688
European commission
CarrerasPathfinders Enabling the next generation in search of blood cancer cures

101126688
European commission
CarrerasPathfinders Enabling the next generation in search of blood cancer cures
PRE2022-102083
Ministerio de ciencia e innovación
REGULACION DE POTENCIADORES DE LA EXPRESION GENICA Y DETECCION DE METABOLITOS POR PARTE DE VARIANTES DE HISTONAS

INVES223200DIES
Fundación científica de la asociación española contra el cáncer
A functional approach to accelerate the development of combinatorial drug therapies in blood cancers

WI 5839/1-1
Deutsche forschungsgemeinschaft
Exploring epigenetic modulation in bone marrow stroma as novel therapeutic approach to prevent leukaemia

101081347
European commission
CarrerasLeaders Carreras Postdoc Program Empowering Future Leaders to Fight Blood Cancers

101108823
European commission
CHROMET Elucidating the role of macroH2A1.2 histone variant as a metabolic sensor in the epigenetic regulation of leukaemia cell fate

146626
Fundació "la caixa"
101108823
European commission
CHROMET Elucidating the role of macroH2A1.2 histone variant as a metabolic sensor in the epigenetic regulation of leukaemia cell fate