OVERVIEW
Lung cancer causes over 1.3 million deaths annually and remains the deadliest type of cancer worldwide. Although efforts in recent years to fully characterize human cancer on a genetic and molecular level have provided important insights to increase our understanding of the gene alteration profile underlying the development of Lung Cancer, the impact of this knowledge in the survival of patients remains modest. Our group is devoted to the genetic, epigenetic and molecular study of the mechanisms that drive LC development. Ultimately, our purpose is to implement the clinical management of cancer patients and to design novel therapeutic strategies.
OUR RESEARCH
The complete genetic characterization of tumours is important to understand cancer development, promote the discovery of new drugs and improve the selection of patients that may benefit from a given cancer therapy. Our research uses the latest high-throughput sequencing technologies to create profiles and catalogues of the recurrently altered genes in cancer. We also have a keen interest in understanding the mechanisms by which the abnormal function of these genes contributes to cancer development.
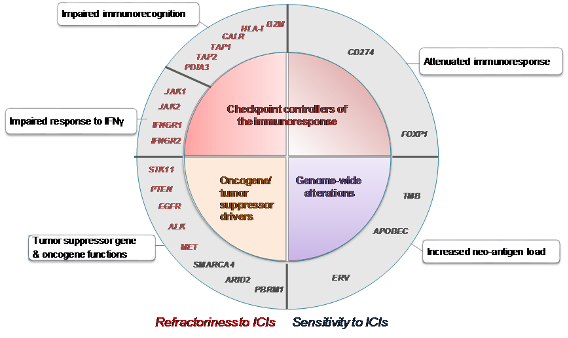

OUR GOALS
Our laboratory is currently engaged in a number of important projects:
1. Screening for factors that determine tumour immunoescape and the response to immunotherapy. We have become increasingly interested in the study of those biological factors, which allow tumours to escape control of the immune system and determine the response to immunotherapy.
2. Genomic and genetic profiling of lung tumours to identify novel targets for therapeutics and determinants for the primary and acquired response to tyrosine kinase inhibitors (TKIs). We use high-throughput genomic sequencing technologies, such as whole exome and RNA-sequencing, to gather information about the genetic background and gene expression profiles of lung tumours from both smokers and non-smokers.
3. Genetic alterations at epigenetic factors: biological understanding and opportunity for novel therapeutics. Over the past 15 years, our group has provided key information to understanding cancer biology. Currently, we are using high-throughput technologies to understand tumour development and to identify molecular vulnerabilities that can be used therapeutically.
OUR CHALLENGES
Recent epidemiological data point to a worrying increase in the incidence of LC in those who have never smoked, particularly women. The reasons are not well understood, a fact that limits the design of prevention measures.
Through our research, we hope to answer the following questions:
1. What are the genetic and molecular abnormalities that trigger the development of cancer, particularly Lung Cancer?
2. How can we use genetic/molecular information to identify novel targets to implement Lung Cancer therapeutics?
3. What is the molecular basis for the lack of response to immunotherapy?
4. How can we predict and prevent acquired resistance to targeted therapeutics?
Team

Montse Sanchez - Cespedes
Senior group leader
Senior group leader

Octavio Alfredo Romero
Research Associate
Research Associate

Xiaoyu Liu
Postdoctoral Investigator
Postdoctoral Investigator

Ana Cristina Díaz
PhD Student
PhD Student

Juan Morillas
PhD Student
PhD Student

Laia Conchillo
PhD Student
PhD Student

Pablo Navajas
PhD Student
PhD Student

Eva Pros
Senior Lab Technician
Senior Lab Technician

Isabel Bartolessis
Senior Lab Technician
Senior Lab Technician
Selected Publications
Current Grants
PID2023-150559OB-I00
Ministerio de ciencia, innovación y universidades
EPILUNG Contribución de las alteraciones en factores epigenéticos al desarrollo del cáncer de pulmón y oportunidades de nuevas vías terapéuticas
RETOS245794PAZA
Ministerio de ciencia e innovación
SOSCLC CÁNCER DE PULMÓN DE CÉLULA PEQUEÑA. DE LAS REDES BIOLÓGICAS A LA TERAPIA PERSONALIZADA
2023 FI-1 00667, 2024 FI-2 00667
Agència de gestió d'ajuts universitaris i de recerca
Estudio de los factores que determinan la evasión tumoral del reconocimiento inmunológico y respuesta a inmunoterapia.

BTVP25S22788
Fundación ramón areces
Nuevas vulnerabilidades moleculares en el cáncer de pulmón de células pequeñas: papel de NOTCH1 y DLL3 en la respuesta a terapias dirigidas